Researchers are exploring ways to manage PsA and help those affected reach new heights on their treatment journey. Learn more about the LATITUDE Psoriatic Arthritis Studies today.
1 https://www.psoriasis.org/about-psoriatic-arthritis
2 Ogdie A, Nowell WB, Applegate E, et al., BMC Rheumatol. 2020;4:2. doi:10.1186/s41927-019-0102-7
1 https://www.psoriasis.org/about-psoriatic-arthritis
2 Ogdie A, Nowell WB, Applegate E, et al., BMC Rheumatol. 2020;4:2. doi:10.1186/s41927-019-0102-7

What if you could potentially impact the 125 million people worldwide (2–3% of the entire population) with psoriasis?1 Embrace the future of psoriasis care with the LATITUDE Psoriasis Studies.
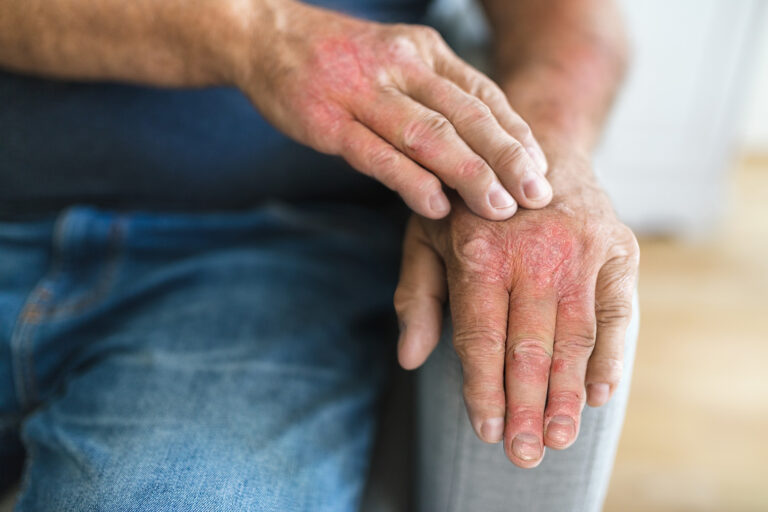
Embrace the future of psoriatic arthritis care with the LATITUDE Psoriatic Arthritis Studies. This program is evaluating an investigational oral treatment for adults with psoriatic arthritis.
The investigational treatment aims to control inflammatory signals known to contribute to the symptoms of psoriatic arthritis. Participants will receive the investigational treatment, an approved treatment (depending on the study you are in), or a placebo (a substance that looks like the investigational treatment but does not contain any active ingredients) while completing various study tests and procedures.
Participants originally assigned to the placebo group will have the opportunity to switch to the investigational treatment during the study. All study treatments will be given as oral medications.

Neither participants nor the study staff will know if participants are receiving the investigational treatment, approved oral treatment, or placebo. Participants should plan to attend up to 14 scheduled study visits over the course of up to 60 weeks.

Rejestracja oznacza wyrażenie zgody na otrzymywanie wiadomości tekstowych i e-mail na temat tego badania. W dowolnym momencie można zrezygnować z subskrypcji. Mogą być naliczane opłaty za wiadomości tekstowe i przesyłanie danych. Oznacza to również wyrażenie zgody na przekazanie Twoich danych osobowych przez firmę StudyKIK ośrodkowi badawczemu w celu określenia, czy spełniasz kryteria kwalifikacyjne do udziału w tym badaniu. Więcej informacji znajduje się w Polityce prywatności i Regulaminie świadczenia usług firmy StudyKIK.
Należy pamiętać, że udział w badaniu jest całkowicie dobrowolny. Jeżeli zdecydujesz się na udział w badaniu, możesz zmienić zdanie w dowolnym momencie.

Durch Ihre Anmeldung stimmen Sie zu, Textnachrichten und E-Mails über diese Studie zu erhalten. Sie können sich jederzeit abmelden. Es können Textnachrichten- und Datengebühren anfallen. Sie stimmen auch zu, dass StudyKIK dem Prüfzentrum Ihre personenbezogenen Daten zur Verfügung stellen darf, um festzustellen, ob Sie für diese Studie infrage kommen. Weitere Informationen entnehmen Sie bitte der Datenschutzerklärung und den Nutzungsbedingungen von StudyKIK.
Bitte beachten Sie, dass die Teilnahme völlig freiwillig ist. Wenn Sie sich für eine Teilnahme an einer Studie entscheiden, können Sie Ihre Meinung über die Teilnahme jederzeit ändern.

Al registrarse, acepta recibir mensajes de texto y correos electrónicos sobre este estudio. Puede darse de baja en cualquier momento. Pueden aplicarse tarifas de datos y mensajes de texto. También acepta que StudyKIK pueda proporcionar su información personal al centro de investigación para ayudar a determinar si es apto/a para este estudio. Consulte la Política de privacidad de StudyKIK y los Términos de servicio con el fin de obtener más información.
Tenga en cuenta que la participación es totalmente voluntaria. Si decide participar en un estudio, puede cambiar de opinión al respecto en cualquier momento.

By signing up you agree to receive text messages and emails about this study. You can unsubscribe at any time. Text messages and data rates may apply. You also agree that StudyKIK may provide your personal information to the research site to help determine if you qualify for this study. Please refer to StudyKIK's Privacy Policy and Terms of Service for more information.
Keep in mind that participation is entirely voluntary. If you do decide to take part in a study, you may change your mind about participating at any time.
We use cookies to optimize site functionality and give you the best experience. Necessary cookies enable core functionality. The website cannot function properly without these cookies and can only be disabled by changing your browser preferences.
For more detailed information on the cookies we use, please check our Privacy Policy.
By continuing to access this website, you are giving us consent to collect cookies.